Open a company in Estonia
The process for starting a company in Estonia includes various actions such as the drafting of the articles of association, as well as the drafting of other documents that include:
- specimen signatures;
- passport copies;
- special forms provided by the company registration office.
Our team of local incorporation experts can assist you through all these steps so that you can start your business in this country as fast as possible.
| Quick Facts | |
|---|---|
| Types of companies |
Limited liability company
|
|
Minimum share capital for Limited Company |
approx. 2500EUR (40.000 EEK) |
| Paid-In Requirement | No, can also be 1eur |
|
Minimum number of shareholders for Limited Company |
1 |
| Time frame for the incorporation |
approx. 5 business days |
| Corporate tax rate |
20% standard tax rate 0% until profits distributed |
| Dividend tax rate |
20% |
| VAT Rate |
20% |
| Are Shelf Companies Available? | Yes |
| Do you supply a Registered Address/Virtual Office? | Yes |
| Local Director Required | No |
| Electronic Signature | Yes |
| Is Accounting/Annual Return Required? | Yes |
| Foreign-Ownership Allowed | Yes |
| Applicable legislation | Company Law. |
|
Legal forms available for foreign companies |
– Subsidiary companies, – branch offices. |
|
Special registration requirements for foreign investors (YES/NO) |
No, there are no specific conditions to be met by foreign entrepreneurs doing business in Estonia. |
| Minimum number of directors | 1. |
| Online registration possibility (YES/NO) |
Yes. |
| Availability of local representative services (YES/NO) |
Yes, you can also use our services for representation with the Estonian authorities. |
| Local address requirements |
Yes, any local company must have a legal address in Estonia. |
| Business incorporation steps |
– Trading name reservation, – documents drafting and filing, – tax registration, – licensing (if necessary). |
| Documents required to open a company |
– Shareholders' identification documents, – company statutory papers, – decision of appointing the manager, – incorporation form. |
| Business licensing requirements (if any) | Yes, for certain domains of activities, such as banking, crypto, and telecom. |
| Possibility to hire local employees (YES/NO) |
Yes. |
| Registration for employment requirements |
Yes, for social security and health insurance. |
| Bank account opening services (YES/NO) |
Yes, we offer corporate bank account opening services. |
| Company documents drafting services (YES/NO) |
Yes. |
| Company formation services availability (YES/NO) | Yes, you can rely on us for full business registration solutions. |
It is important to keep in mind that the process of company formation in Estonia requires that the procedures must be performed through a public notary. Setting up a company in Estonia requires also opening a bank account, hiring a local accountant and finding a registered office in Estonia.
Our affiliations
CompanyIncorporationEstonia.com is partner of BridgeWest and a proud member of several well-known web portals, such as the web portal Lowtax.net.
Types of companies in Estonia
Osaühing (OÜ – private limited company). At least one member is necessary in order to start the Private Limited Company in Estonia and a minimum share capital of 40.000 EEK divided into shares (with a minimum value of 155 EUR per share). The shares of this type of business established in Estonia cannot be freely traded to the public unlike the shares of the public limited liability companies. The contribution of each shareholder is deciding the liability of each member.
A Public limited liability company in Estonia (Aktsiaselts-AS) is the most popular form for medium and large sized companies who can provide a minimum share capital of at least 40.000 EEK. The shares of such a company can be freely traded to the public by registering it at the Stock Market.
At least two individuals or corporate bodies can be united under the same economic purposes after they sign an agreement. This form of Estonian business is called General Partnership. For this business, no minimum capital is necessary and all the partners have full liability and can equally take all the management decisions. The profits are shared between the partners.
In an Estonian Limited Liability Partnership, the liability can be limited (in case of a silent partner) or can be unlimited (in case of a general partner). Just like in the case of the general partnership, there is no requirement for a minimum capital. Only the general partner can be a manager of this type of entity.
In Estonia is also possible to open a business where the owner doesn’t need to deliver a minimum share capital and whose liability is unlimited on the company’s debts and profits. This is the Sole Proprietorship. Our incorporation agents can help you open a company in Estonia, no matter if you choose to register an LLC or a sole trader.
The Private Limited Liability Company is the most chosen form of a company by which investors prefer to operate in Estonia and the incorporation procedure for this type of company is not difficult, due to an online platform established by the Estonian state.
An investor may also choose to buy a Shelf Company in Estonia, as the business is already registered and, by a power of attorney, the new owner can immediately begin operation.
If you intend to open a company in Estonia or you have already opened one, you might find useful our tax calculator, a tool which shows you the amounts you have to pay as corporate tax, dividend tax and VAT in Estonia.
Also, if you are interested in setting up a company in another European country, for example in Ireland, our partner law firm can provide you with assistance.
Company formation requirements in Estonia
Both foreign and local entrepreneurs who want to open a company in Estonia must comply with the Company Law. According to the legislation, the following requirements must be fulfilled:
- choose an appropriate business name, which must be unique, and reserve it with the Trade Register;
- provide the passport and other personal information to the Companies Registrar;
- have the company’s Articles of Association drafted and notarized before filing them for registration;
- obtain a legal address for the company (virtual office services can be offered on request);
- open the company bank account for the share capital to be deposited.
Our Estonian company formation representatives can help in preparing all the documents related to setting up a business in this country. If you need reliable legal assistance in this country, we can put you in contact with our experienced Estonian lawyers who offer a wide range of legal services. In case you need debt collection services in another country, for example in Germany, we can put you in contact with our partners.
Steps for company registration in Estonia
In order to open a company in Estonia, a foreign investor should complete the following steps:
- choose a business form which suits with the needs of the investor (most of the times, the limited liability company is selected);
- the company’s statutory documents must be drafted and notarized by a public notary, if any documents need to be translated, these should also be notarized;
- the documents are then filed with the Companies Register in Estonia electronically, in person or by proxy, in accordance with the client’s request;
- registering with the Estonian tax authorities, which will issue the tax identification number, the VAT number and will approve the registration for social security purposes;
- applying for the licenses, permits or approvals, as required by the authorities in the field the company will operate in.
- The last step is registering an Estonian company with the Central Sick Fund of Estonia for health insurance. All the new employees must be registered within one week of their employment.
In case the company will complete trading activities, it must also register for VAT and with the Customs Authorities in Estonia.
Foreign investors from non-EU countries interested in setting up startup companies can apply for the start-up visa which enables them to come to Estonia and register their businesses here. If you are interested in starting a business in Estonia, our specialists can help you.
Costs for registering a business in Estonia
Before starting the company formation procedure of an Estonian business, we also present you the costs associated with such an action:
- the business registration fee imposed by the Trade Register’s offices in Estonia is set at around 145 EUR for the regular registration and 190 EUR for a quick registration;
- the virtual office cost is established at approximately 99 EUR per month – the virtual office represents a great advantage for those seeking to register companies remotely;
- the minimum share capital which needs to be considered for registering a limited liability company in Estonia is 2,500 EUR;
- our company formation fee for a new company is of around 850 EUR, and our local agents can explain what this cost is comprised of;
- the accounting costs start at approximately 100 EUR per month as a basic fee, and we invite you to contact us if you need additional services.
Our Estonian accounting company can assist local firms in adhering to the tax laws. Our accountants can offer clients a wide range of services, including audit, bookkeeping, payroll, tax, and other related ones. By outsourcing a number of activities to our company, business owners can maintain a clear understanding of their financial condition and concentrate on the important aspects of their operations.
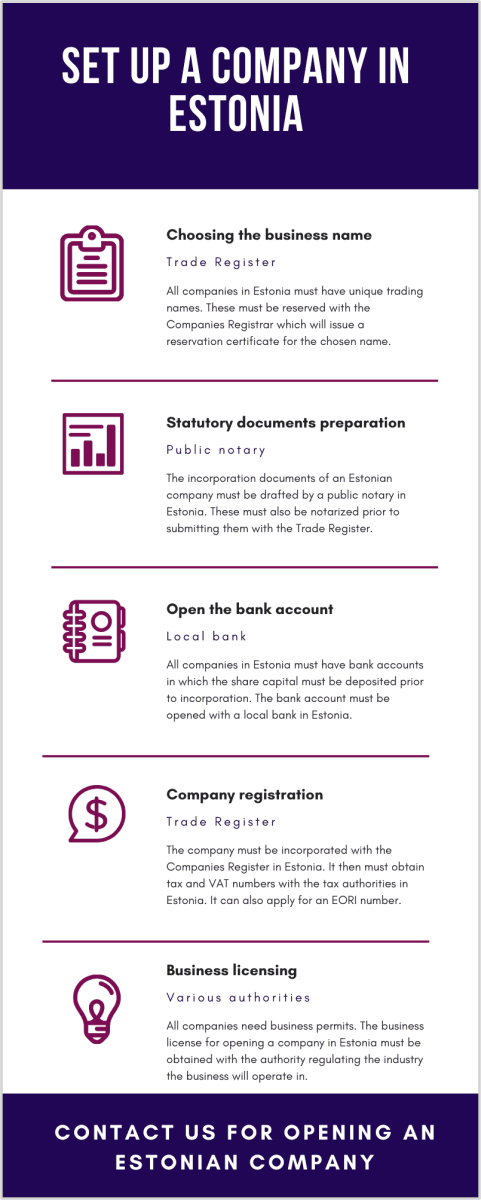
We also invite you to see our scheme on how to open an Estonian company:
Business name reservation in Estonia
Once the type of structure has been selected, the first procedure to complete is to reserve a business name. The selected name must comply with the provisions of the Company Law and must be unique. In order to ensure a successful trading name reservation, the future business owner is advised to submit at least 3 names for approvals, among which there should also be the desired one.
The trading name reservation procedure is the simplest and takes no more than a few hours to be completed and the trading name reservation certificate to be released. The business owner is also required to provide an e-mail address upon the reservation of a trading name in Estonia.
Legal address requirements when starting a business in Estonia
One of the main requirements for setting up a business in Estonia is to have a legal address here. This will determine the residency of the business in terms of operations, but also taxation. Th legal address can consist in a rental contract for a traditional office space or a virtual office that can be used in order to complete the incorporation procedure faster.
Our company registration specialists in Estonia can provide virtual office services at very advantageous conditions in large cities in the country.
Preparing the company’s statutory documents
Based on the type of company to register, there are various documents that must be submitted for approval and verification with the Estonian Trade Register. Among these, the shareholders must provide their personal information if they are natural persons. In the case of foreign companies setting up branches or subsidiaries in Estonia, these are required to file extracts from the registrars in their home country alongside other information. Considering most structures are required to have a management board made of directors, their personal information must also be provided.
The information mentioned above must be provided no matter the business form selected. Then, in the case of setting up a sole trader or partnership, a simple registration form must be completed and filed with the Companies Registrar in the case of the sole proprietorship, while in the case of a partnership the legal agreement which provides for its creation must also be submitted.
In the case of complex structures such as limited liability companies, the Articles of Association must be drafted and notarized by a public notary in Estonia. These must contain specific information about the shareholders, the directors, the capital of the company and the distribution of shares among the stockholders.
Our local advisors can help with the preparation and filing of the necessary documents for starting a company in Estonia.
Bank account opening in Estonia
It is a mandatory requirement for a resident company to have a bank accountwith an Estonian bank. The account will first be used to deposit the share capital and after the company is incorporated it will be used for completing the financial operations of the business.
If you want to create a company in Estonia, for example if you plan on establishing an e-commerce company in this country, we can assist with the opening of corporate and merchant bank accounts.
Tax and VAT registration in Estonia
One of the main steps of starting a business in Estonia is tax registration. This process is usually completed right after the company is registered with the Trade Register. It must also register for social contributions as an employer.
Then, the company can register for VAT voluntarily. It is important to note that when the goods and services supplied exceed 16,000 euros, VAT registration becomes mandatory in Estonia. This applies to both natural persons and companies making supplies of VAT-taxable goods and services.
Business licensing in Estonia
The last step before commencing the activities under a business incorporated in Estonia is to apply for the licenses required to completing one or more undertakings. Estonian companies must apply for such permits with the governmental agencies, ministries or other authorities in charge with regulating the industries these companies will operate in.
In the case of trading companies, both VAT and EORI numbers are required in order to complete import-export activities in the European Union.
If you intend to create a company in Estonia and you need information or have any questions about the business permits and licenses required to start a company in Estonia, our representatives can offer the necessary information.
Investors who intend to launch businesses in Estonia in the cryptocurrency industry should be aware that they will be subject to the rules that the National Bank impose. You can enquire about the rules enforced when establishing a crypto business from our company formation consultants. It’s important to note that certain companies must now register and obtain a crypto license in Estonia from the National Bank.
How long does it take to register a company in Estonia?
Those who want to open a company in Estonia must consider the following timeline:
- the name reservation takes less than one day and can be completed online;
- filing the company’s incorporation documents will take one day, but can also be completed online;
- tax and VAT registration is completed online and can take about 3 days;
- registering as an employer with the Employer Registry takes a few hours.
We remind foreign investors that they can register companies remotely by obtaining e-residency in Estonia. Our local agents can offer more information on this aspect.
FAQ on opening a company in Estonia
The number of shareholders in an Estonian company starts at one. They can be natural persons or other companies.
With the new e-residence regulations, opening a company in Estonia is easier than ever. With the help of our advisors in Estonia, this procedure can be simplified.
The registration process of an Estonian company can take up to one week. However, the investor must also consider the licensing procedures which depend on the industry the company will operate in.
The costs associated with starting a business in Estonia depend on various factors, such as the type of company established, the share capital requirements and the fees related to drafting the incorporation documents.
The corporate tax in Estonia is set at 20%.
Estonia as a business destination
Estonia is a well-developed country with good infrastructure and a competitive business environment. An important number of foreign investors has chosen the country to invest in areas such as communications and IT. In Estonia, foreign investors enjoy the same rights as local ones but they also need to observe specific rules and regulations for doing business. Moreover, the Estonian government encourages foreign investors to come and open a company in Estonia through incentives and a friendly business environment. In addition to that, it offers a liberal trade, a balanced budget and favorable legislation for starting a company in Estonia.
The Estonian economy brought Estonia on the 25th place as far as the ease of starting a business by The World Bank Group is regarded. Foreign investors play an important role in the economy of Estonia, especially through investments in fields like telecommunications and banking. Also, due to its location, Estonia developed important relationships with countries like Finland and Russia in terms of international trading.
Estonia has an excellent geographical position in the EU and has always taken advantage of it. Situated within an important trading route between eastern and western Europe, the country has important ports and the trade sector is very well developed here.
What to consider when starting a business in Estonia
Both local and foreign entrepreneurs benefit from similar conditions and requirements when opening companies in Estonia, however, there are various aspects which need to be considered individually by investors. Among these:
- researching the market and the industry, or sector the business will operate in Estonia;
- choosing the appropriate business form the investor will use to offer his/her services;
- deciding on whether the company will be managed from or outside Estonia (e-residency is available here);
- researching the licensing requirements in accordance with the Estonian industry to operate in;
- considering the minimum amount of money to start the operations, as well as the legal requirements in this sense;
- registering the company with the Estonian Trade Register and tax authorities in the country.
The steps for opening a company in Estonia can be explained by our local consultants. Companies must also complete the Estonia VAT registration process.
Starting an Estonian business through e-residency
EU citizens have two main options when it comes to starting a business in Estonia: the usual way which implies relocating here and managing their companies from within the country, or e-residency which enables them to remain in their home countries while owning companies in Estonia. In the last few years, the second option became very popular among foreign investors and not only because of the facilities it offers but also because of the other many advantages the Estonian government has enabled.
Setting up a company in Estonia by becoming an e-resident of this country implies obtaining a digital ID by registering on the government’s website dedicated to e-residents.
All categories of investors can set up companies this way in Estonia, from simple freelancers to complex businesses which allow remote management.
If you want to open a company in Estonia by obtaining e-residency, our local advisors will guide you through the entire process.
Why start a business in Estonia?
In case you want to create a company in Estonia you should know that ever since Estonia introduced the e-residency program, it has registered a substantial economic development. In numbers, Estonia looks like this:
- 98% of the companies registered here have been incorporated online;
- 99% of the banking transactions completed here are online;
- 95% of the annual tax documents are filed online;
- you can register a company online in 3 hours;
- in 2018, Estonia occupied the 1st position in the EU Digital Economy and Society Index;
- in 2018, Estonia had the largest number of startups in the European Union.
Our company formation representative in Estonia can help you enter your company in the Commercial Register and can offer you assistance for doing business in Estonia. Please contact our experts for more detailed information and advice.